Scotland: new approval pathway for ultra-orphan medicines
People in Scotland with rare diseases may be able to access new treatments in a more efficient manner following the introduction of a new definition of ‘ultra-orphan medicines’.

UK campaign for access to treatments and support for those with spinal muscular atrophy
People in Scotland with rare diseases may be able to access new treatments in a more efficient manner following the introduction of a new definition of ‘ultra-orphan medicines’.

Cytokinetics announced results of a recently completed clinical trial of reldesemtiv in SMA. The data suggest that this new experimental drug for SMA increases endurance and respiratory function even over a short period of 8 weeks.
Cytokinetics announced results of a recently completed clinical trial of reldesemtiv in SMA. The data suggest that this new experimental drug for SMA increases endurance and respiratory function even over a short period of 8 weeks.
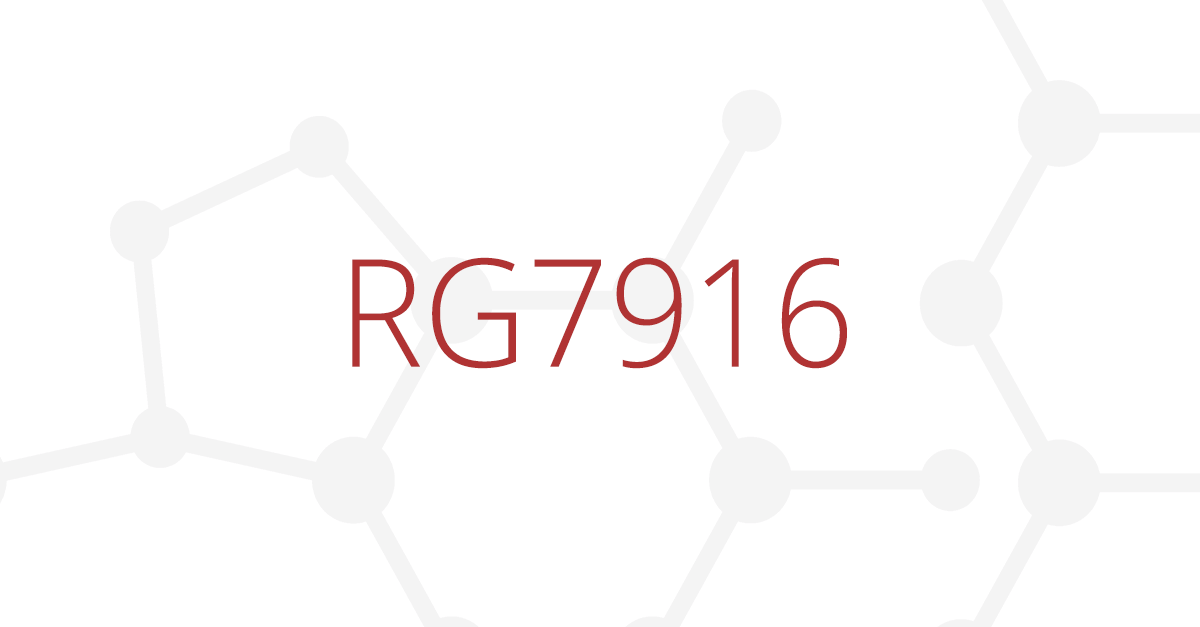
TreatSMA has learned that UK will not take part in the SUNFISH trial
TreatSMA has learned that UK will not take part in the SUNFISH trial

Recruitment for the SUNFISH clinical trial is about to be completed globally. As of last week, enrollment of participants aged 6–11 and 18–25 years has been completed. But the UK red tape has blocked the sites from opening on time.
Recruitment for the SUNFISH clinical trial is about to be completed globally. As of last week, enrollment of participants aged 6–11 and 18–25 years has been completed. But the UK red tape has blocked the sites from opening on time.

NICE Appraisal Committee will meet on 27 June to discuss draft recommendation for Spinraza provision in England and Wales. You are welcome join!
NICE Appraisal Committee will meet on 27 June to discuss draft recommendation for Spinraza provision in England and Wales. You are welcome join!