
Community response to NHS letter
Today TreatSMA sent a reply to the NHS letter received last month that defended the draconian funding restrictions for compassionate access to nusinersen in spinal muscular atrophy (SMA).

UK campaign for access to treatments and support for those with spinal muscular atrophy

Today TreatSMA sent a reply to the NHS letter received last month that defended the draconian funding restrictions for compassionate access to nusinersen in spinal muscular atrophy (SMA).
Today TreatSMA sent a reply to the NHS letter received last month that defended the draconian funding restrictions for compassionate access to nusinersen in spinal muscular atrophy (SMA).

Dear Community, After we all partook in sharing the TreatSMA video to raise awareness of SMA, we have been flooded with many questions about how can the community help. The fact is: NHS will not pay this much money for any drug voluntarily. We have between 1–2 thousand SMA-ers in the UK. Which means that…

Dear Community, After we all partook in sharing the TreatSMA video to raise awareness of SMA, we have been flooded with many questions about how can the community help. The fact is: NHS will not pay this much money for any drug voluntarily. We have between 1–2 thousand SMA-ers in the UK. Which means that…
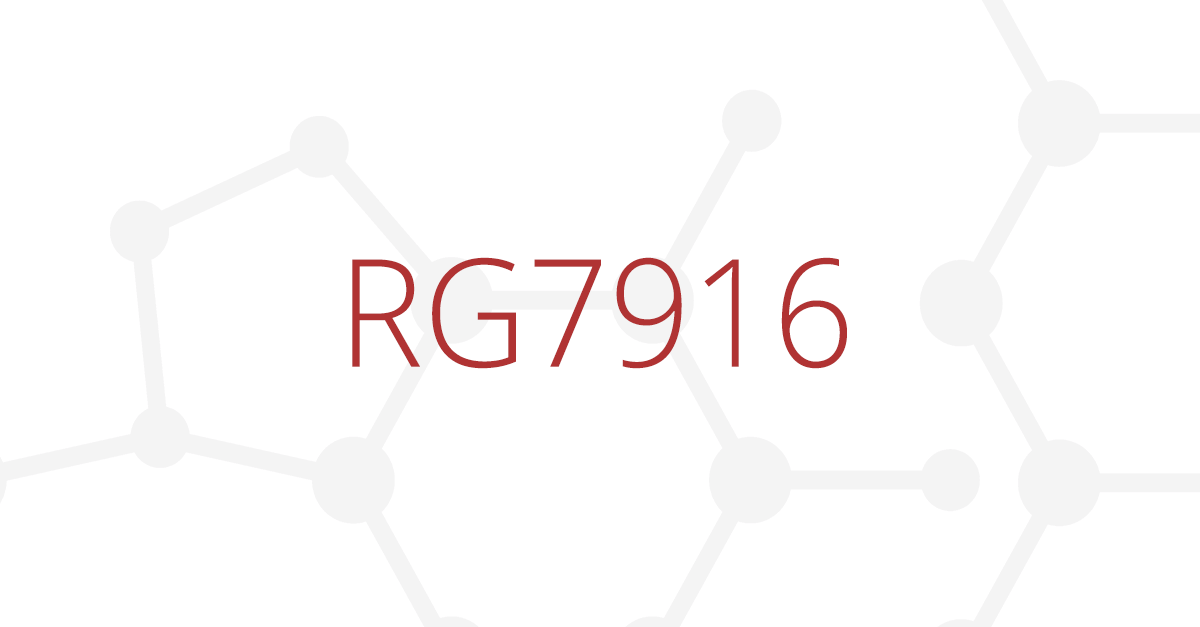
PTC Therapeutics informed that the experimental drug RG7916 increased the SMN protein 2.5-fold in SMA type 2 and 3 patients who took part in the SUNFISH clinical trial.
PTC Therapeutics informed that the experimental drug RG7916 increased the SMN protein 2.5-fold in SMA type 2 and 3 patients who took part in the SUNFISH clinical trial.

TreatSMA has received a formal response from NHS England to the letter sent by the UK medical community about the Expanded Access Programme of nusinersen.
TreatSMA has received a formal response from NHS England to the letter sent by the UK medical community about the Expanded Access Programme of nusinersen.

Today 30/9 the world celebrates SMA Awareness Day. Do you know what SMA stands for?