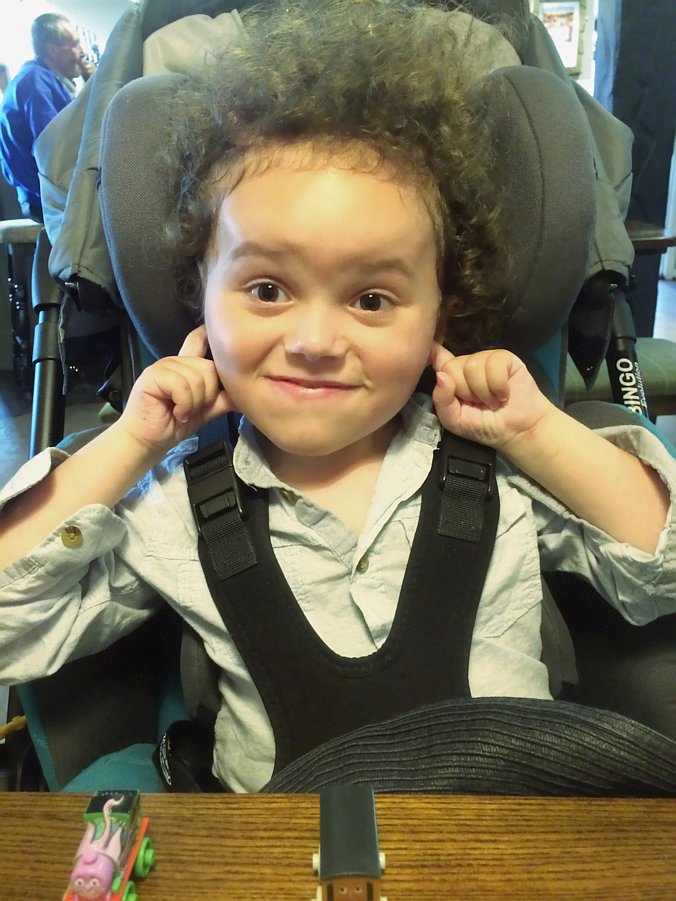
TreatSMA press release
Every month, across the UK, parents are hearing the devastating words from doctors that their baby is extremely unlikely to survive to see their 2nd birthday. That they will helplessly be forced to watch as their baby grows weaker before their eyes, losing their ability to move their legs; their arms; to lift their head; to…